-> HathiTrust Digital Library Site; Link to First page of article.
The framed pics was not part of the source, I added them from my collection
The somewhat sudden discovery and rapid development of the Cripple Creek district in Colorado soon resulted in a large output of tellurium gold ores of too low grade to stand the heavy transportation and smelting charges, which varied before the railways reached the camp from $15 to $18 per ton.
Stamping and amalgamation having early proved a failure, the economic treatment of these somewhat peculiar ores presented a fruitful field of experimental research which for some time engaged the attention of many metallurgists, offering as it did an instructive and profitable field for the introduction of chemical methods of gold extraction.
After much experimenting with the well-known and tested processes during the summer and fall of 1893, I came to the conclusion that cyanide of potassium was the best all-round solvent for the gold in the Cripple Creek low-grade ores.
Much remained to be done, however, in the way of perfecting the mechanical treatment of the ore in order to produce what we then thought necessary, namely, "a fairly even and granular material, as free as possible from dust when dry-crushing was practiced, and equally free from slime if crushed wet."
Early in 1894 I had the opportunity to put my ideas into practical and successful operation in a small cyanide mill which had shut down after an unsuccessful run, while during the summer I was engaged by local capitalists to report generally on the treatment of Cripple Creek ores in view of the contemplated erection of large works; and later in the same year was engaged to erect and manage the works which form the basis of this paper.
Before describing the works, however, it may be in order to briefly glance at the ore it was erected to treat.
The ores of Cripple Creek, Colo., are altered andesite, granite or phonolite, containing thinly disseminated iron pyrites and tellurium minerals, mostly calaverite, associated with quartz and very often fluorite.
At and near the surface the tellurium is oxidized, and the gold when visible exists as an ocher-color powder (mustard gold) or semi-coherent mass, very often retaining the form of the tellurium crystal from which it was liberated.
The pyrite carries gold, but not to any great extent.
Free gold also exists in the unaltered phonolite dikes, very often in paying quantities.
The thoroughly oxidized ores are quite amenable to cyanide, while the "blue" unoxidized ores require roasting before they will yield a satisfactory extraction, for the reason that the tellurium minerals, calaverite and sylvanite, are all but insoluble in 0.5% cyanide solutions, while by roasting the tellurium is oxidized and the gold set free in the metallic state, easily soluble in mill solutions.
The works of the Metallic Extraction Co. are situated at Cyanide, a station on the Florence & Cripple Creek Railway, about 35 miles from the Cripple Creek mines and two miles from Florence, the oil center of Colorado.
In this part of the valley of the Arkansas coal and oil are cheap and abundant, labor plentiful, and indeed all the conditions incident to the building up of a large metallurgical enterprise are at hand.
The works are erected on a flat site, following out my often expressed opinion that a flat site is preferable to a slope or terrace, since on the former works can be laid out not only to the best advantage but also can be operated at less expense.
The ore reaching the works from the Cripple Creek mines is delivered by the railway company on a double-track trestle, this trestle at the sampling works being 20 ft. above the floor line of the works.
There are two sampling works, each of a capacity of 200 tons per day.
No. 1 consists of one No. 4 Gates crusher and two No. 1 Gates crushers with intermediate screens. The ore is here crushed to about 1 in. cube, elevated to a Vezin sampler, which cuts out 25% and diverts it to crushing rolls, which reduce the ore to about 4-mesh.
It is then elevated and passed over another sampler, which cuts out 0.1 part of the 4-mesh sample or 2.5% of the original ore. This cut is sent to the fine-crushing rolls and worked down in the usual manner for the sample, which in the final pulp is reduced to pass a 120-mesh sieve.
The sampling machine takes 44 cuts per minute from the stream of free falling ore, traveling horizontally through the entire stream for each cut. The main part of the ore is carried off by special conveyors and distributed automatically in the storage bins.
No. 2 sampling works consists of a 12x20 in. Monarch crusher and two 30x5 in. Blake Challenge crushers. The ore is shoveled from the railway cars into a chute leading to the Monarch, while all of it but 2.5%, cut out for the sample, is automatically conveyed to the storage bins in front of the fine-crushing mills.
This ore is thus handled but once during the coarse crushing, sampling, and distribution in the storage bins. These bins have a capacity of 1,500 tons.
Ores are received in the sampling works from all parts of the Cripple Creek district, and are purchased outright from the mine owners on the basis of its assay value.
Ores under 2 oz. are at present purchased at $19 per oz. for the gold, less $7 to $11 treatment charge, which treatment charge includes the freight from the mine to the works.
For ores containing over 2 oz. $20 per oz. is at present paid for the gold, less $10 to $14 per ton freight and treatment charge.
The ore is brought from the storage bins into the fine-crushing mills by belt conveyors; here the first step is to drive off the moisture and raise the ore to such a temperature as will insure good screening. It is not alone sufficient to drive off the moisture, since it has been found that the hotter the ore the greater the capacity of the machines.
There is, of course, a limit to heating the ore, depending largely on the elevators used; if these are of ordinary rubber belting the limit is soon reached; iron elevators, however, give ample margin.
The dryers used are of the tubular pattern. The No. 1 dryer consists of four steel tubes nested together inside two track-bands and connected at the feed and discharge ends by two hoods. The tubes are lined with fireclay tile and revolve as one cylinder.
It will be noticed that the ore is divided into four thin streams and brought into intimate contact with the heated air and gases passing through the 18-in. tubes of the dryer.
The motion of the ore advancing in these small tubes is very gentle and regular, and consequently produces but little dust. An improvement in the single cylinder dryer consisted in dividing it into quadrants by plates placed at right angles extending longitudinally through the cylinder.
This improvement increased the drying efficiency of the cylinder at the expense of a higher dust loss and greater wear and tear, as the ore is alternately sliding along the plates from the center to the periphery and back to the center again during a revolution of the cylinder.
By using four or more small tile-lined cylinders instead of one large cylinder divided into quadrants I succeeded in again increasing the efficiency and reducing the dust loss and wear at the same time.
It will be noticed that this tubular dryer is always in balance, the ore in an ascending tube being balanced by that in a descending one, or very nearly so.
The capacity of No. 1 dryer is 80 to 100 tons per day. Image at right shows our No. 2 dryer while in process of erection. Its capacity is 180 to 200 tons per day.
The ore, deprived of its moisture and heated to about 300° F., is taken up by steel bucket elevators and delivered to the coarse screens, the oversize returning to a set of 36-in. crushing rolls, by which it is reduced to about 8-mesh, while the ore passing the mesh of the coarse screens is delivered to the next finer screen.
The crushed ore is again elevated, screened, and the oversize conducted to a set of 15x26 in. crushing rolls, which reduces it to about 15-mesh; and the screened oversize from this roll is divided between two finishing rolls of the same size and construction.
The fine ore is taken out after each passage of the material through the rolls, and indeed any pulp of the desired mesh existing in the uncrushed ore is screened out directly without passing through the crushing rolls.
We govern the peripheral speed of our rolls according to the size of the material they receive, as follows:
- Rolls receiving up to 1.25 in. cube, 350 to 400 ft. per minute
- Rolls receiving up to 0.25 in. cube, 550 to 600 ft. per minute
- Rolls receiving up to 14-mesh, 700 to 750 ft. per minute.
Finishing rolls receiving material from 15 to 20-mesh for reduction to say 40-mesh can be driven at speed of 1,000 ft. and over; indeed the speed limit will under these conditions be found in the journals and not in the size of the ore particles. I am not, however, an advocate of speeds much higher than 1,000 ft. per minute.
Fine crushing rolls should be supplied with a regular and heavy feed, so that the ore can be, to a large extent, crushed upon itself. In this way the maximum crushing capacity can be attained while the efficiency of the rolls is not reduced by slight inequalities or irregular wear of the shells.
For fine crushing, massive and well-built rolls are a prime requisite. The journals should be outside of the dust-proof housings, well protected against dust and grit and amply provided for thorough lubrication.
For 26x15 in. rolls the shafts should be not less than 7 in. diameter in the journals, and the boxes should be of the ball and socket variety, the whole figured for a steady working-spring pressure of 15 tons on each side, or pair of journals, and capable of standing up under double this pressure for intervals.
The Metallic Extraction Co. has three fine-crushing mills;
Mill No. 1 has one 27-in. and three 26-in. crushing rolls, with a capacity of 100 tons per day to 40-mesh.
Mill No. 2 has one 36-in. and three 26x15 in. rolls, its capacity is 170 tons per day to 30-mesh.
Mill No. 3 is similarly equipped, but embodying experience gathered from the former work has a capacity of 200 tons per day to 30-mesh.
The tonnage, however, gives but a poor idea of the mass of stuff ground up in one of these mills during each 24 hours. The ore averages in bulk as it reaches the works in railway cars 25 cu. ft. per ton; when reduced to 30-mesh it averages 23 cu. ft. to the ton, consequently a mill crushing 200 tons per day of this very light material has to dispose of no less than 5,000 cu. ft. of ore every 24 hours.
Mill No. 1 has so far been used exclusively for crushing oxidized ores, which are sent directly from the mill to the leaching tanks. The supply of this class of ore is now very small and uncertain, so much so that with the completion of our new roasting furnaces we propose to roast all ores coming to the works.
Mills No. 2 and 3 are under the same roof, and as the process is entirely automatic three men on a shift run both mills. These mills are situated adjoining the roasters and treat exclusively ores that are subsequently roasted. Between the mills and the roasters storage bins are provided for 800 tons of crushed ore.
The first roaster put in was a straight-line mechanical furnace. Afterward six tubular roasters were added, bringing up the roasting capacity to 400 tons per day. The sulphur in Cripple Creek ores now averages about 2%, and we procure satisfactory extractions by roasting down to 0.1% sulphur. It requires, however, a very high terminal heat to break up the sulphates.
This, in cyanide treatment, is the crucial point, and we find the rotary tabular furnaces give the desired roast with scarcely a trace of sulphate left in the product.
The temperature, however, necessary to obtain these results comes very near the sintering point of the ores, and owing to the small diameter of the tubes the heat from the fire-box is so concentrated in the hood at the discharge end of these furnaces that there is no difficulty in sintering the ore if necessary; our aim is to bring the heat close to the sintering point.
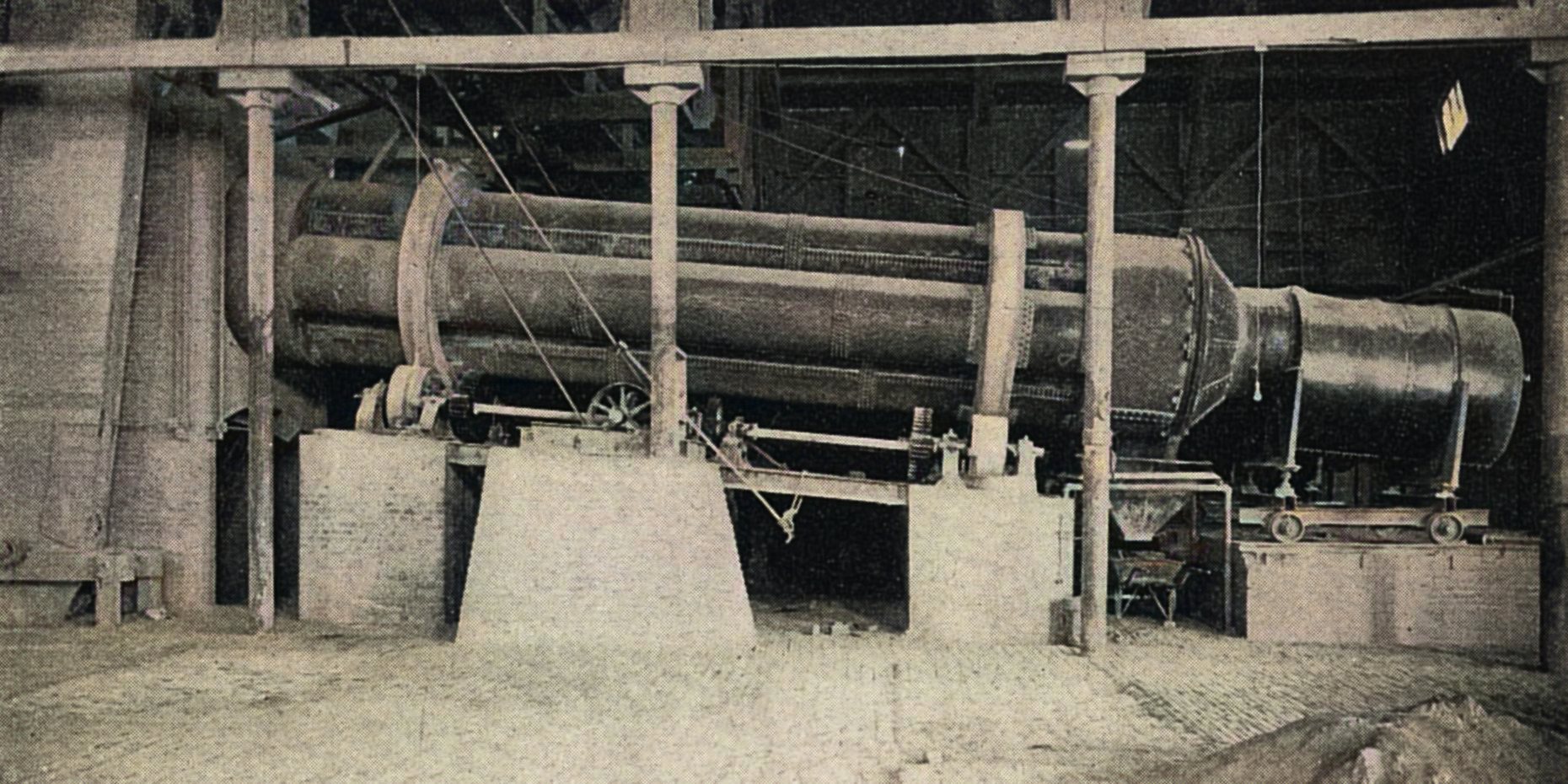
The tubular roasting furnaces are of the same general design as the dryers; they are, however, built much stronger. The discharge is also different.
In the standard roaster the tubes are 29 ft. long by 25 in. diameter inside the lining. They make one revolution in 4.8 minutes and average 48 tons per day from 2% to 0.1% sulphur.
The roasted ore is discharged continuously through a series of openings in the periphery of the hood (Image at left). All these except the two over the ore-hopper at any one time are covered with a band of iron which prevents the exit of flame around the top of the furnace.
The fire-box is built of steel plate and mounted on wheels. The furnace is driven with friction wheels at each track band, operated through a differential drive which insures an absolutely even motion.
These furnaces are patented in the United States and abroad.
The roasted ore is discharged into conveyors traveling over water-cooled surfaces, so that by the time it reaches the leaching-room the ore is ready to be charged into the tanks. This system of automatic cooling has been in use since September, 1896, and has given perfect satisfaction up to its full capacity.
Much has been written concerning the loss of gold in roasting telluride ores. Our experience in the treatment of nearly 100,000 tons of these ores shows but little loss of precious metals. The conditions incident to the successful roasting of tellurides are, a very gentle and slow application of heat, such as can be attained in a furnace fired at one end, or better still in a tubular furnace.
In the tubular roasters used in these works we find the best results from a three hours' exposure of the ore in the furnaces; during the first two hours the ore is gradually advanced to a dull red heat and for the last hour it is subjected to a good roasting heat, terminating at a bright red, close to the sintering point.
The tanks in use at the Metallic Extraction Co.'s works are built of steel; the last added are 50 ft. in diameter and hold 450 to 500 tons. The charge, when extraction is completed, is sluiced out through bottom discharge valves and pumped to the dump by centrifugal pumps.
The filters used are cane matting supported on a wooden framework and covered with 10-oz. duck. The solution passing these filters is perfectly clear, provided the ore is properly roasted. With badly roasted Cripple Creek ore, sulphates of magnesia and alumina prove very troublesome, inasmuch as the hydrates precipitate on exposure of the solution to the air, and also in mixing with solutions flowing from other and more alkaline charges, coating the zinc with a gelatinous film which effectually prevents the precipitation of the gold.
Zinc in the form of shavings, and also zinc dust, is used to precipitate the gold; the latter we believe preferable when adapted as a continuous process, more especially as the action is quicker and less zinc is required per ounce of bullion precipitated.
The method of precipitation by means of zinc has been very often criticized, but nevertheless it has stood the test of time, and for solutions running from 0.05% of cyanide upward, and low in gold, it is likely to continue for a considerable time to come the best precipitant of gold from cyanide solutions, particularly when used in the form of dust.
I do not wish to be understood as stating that solutions below 0.05% in free cyanide and low in gold cannot be economically treated by the zinc method. I do say, however, they become more trouble-some, require much longer contact with the zinc and greater care to obtain uniform results; therefore to this strength at least electrical methods have a fair field; for solutions carrying over 0.1% cyanide I cannot see the slightest chance for electrical precipitation to compete with zinc as we use it here.
The presence of zinc is claimed by some to be very detrimental to the solvent power of cyanide solutions. This is not quite correct, since zinc does not accumulate to any great extent in the solutions. The highest I have ever found is 0.55% in our strong solution, while the average for over two years is but 0.312%.
To determine what effect mill solutions had as compared with pure solution, I caused 42 tests to be made with various ores received at these works, 21 with ordinary mill solution containing zinc and 21 with pure solution of potassic cyanide.
Taking the extraction given by the pure solution as 100%, I found mill solutions give on blue ore (raw) 96.6%, oxidized (raw) 100%, and roasted ore 95.1%. The consumption of cyanide, however, was 25% less in the mill solutions than in the pure solution.
To fully test this point I had 36 further tests made on three different classes of ore under precisely similar conditions, 18 with mill solutions, and 18 with pure solutions.
Here again the zinky mill solutions showed 28% less cyanide consumption than the pure solutions. The solutions were titrated with silver nitrate, making the usual precautions in the case of the mill solutions.
I do not claim scientific accuracy in determining the free cyanide in the mill solutions in the presence of zinc, but I believe the tests are fairly accurate.
It has often been noticed that the laboratory tests on ores almost invariably give a higher cyanide consumption than is found to occur in the actual treatment. The cause of this discrepancy is, I think, clearly shown by the foregoing experiments, since realizing this fact we make all our laboratory tests with mill solutions, which check up very closely with the mill work, both as to time of treatment for a given ore, and also the consumption of cyanide.
Four years ago I was led to the conclusion that the zinc was chiefly precipitated in the charges of fresh ore in the lixivation tanks.* Since then I have proved the correctness of this view, and it would appear from the foregoing tests that at least part of the cyanide combined with the zinc is available for dissolving gold, probably after the reaction has taken place which results in throwing down the zinc and regenerating potassic cyanide.
This interesting study I hope to resume shortly.
My present views are;
1) that mill solutions will give equally as good extraction as pure solutions, but they require longer contact with the ore. say 10% longer.
2) mill solutions will give the same extraction as pure solutions, with about 25% less consumption of cyanide.
3) the lower consumption is probably in part due to the mixed cyanides in mill solutions being less sensitive to cyanicides and partly to the potassium, sodium or other cyanides regenerated after the zinc is precipitated in the ore.
I have at various times attempted to determine the reactions that occur in the zinc precipitation boxes, more particularly to determine the conditions incident to good precipitation of the gold. The results of my investigation have, however varied so widely with minute changes in the composition of the solution that I could not obtain concordant results in all particulars, though I have secured much interesting information that serves a useful purpose in our work.
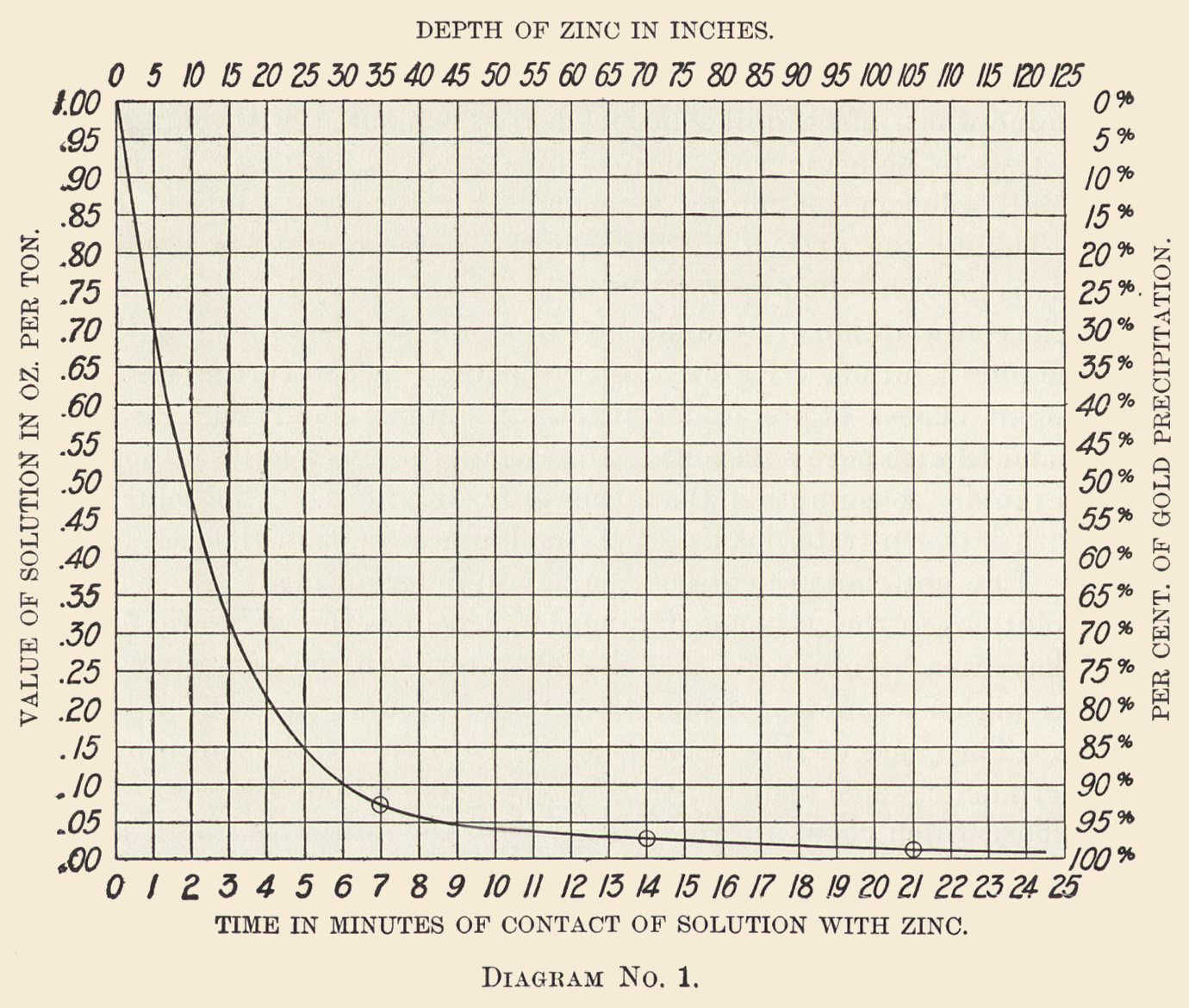
Diagrams Nos. 1 and 2 are plotted from determinations made during six continuous days. The zinc boxes were here filled with shavings pressed down to the density we find best for the purpose. Accurate determination showed that the zinc occupied but 3% of the space, leaving 97% space available for the passage of the solution.
The zinc boxes are 4 ft. wide, the solution flowed alternately downward through the zinc shavings in one compartment and upward in the next, overflowing into the third compartment and so on; at the point of overflow from each double compartment a continuous sample was drawn off through a glass tube, the position of which was changed from time to time so as to take part of the stream across its full width.
Continuous samples were so drawn from each compartment and the determination made every 12 hours. These results were averaged and plotted.
Diagram No. 1 shows the gold precipitation as deduced from these experiments, the depth of zinc in inches through which the solution passed, and the percentage of gold precipitated at each point. It may be taken as normal precipitation with solutions in good working condition.
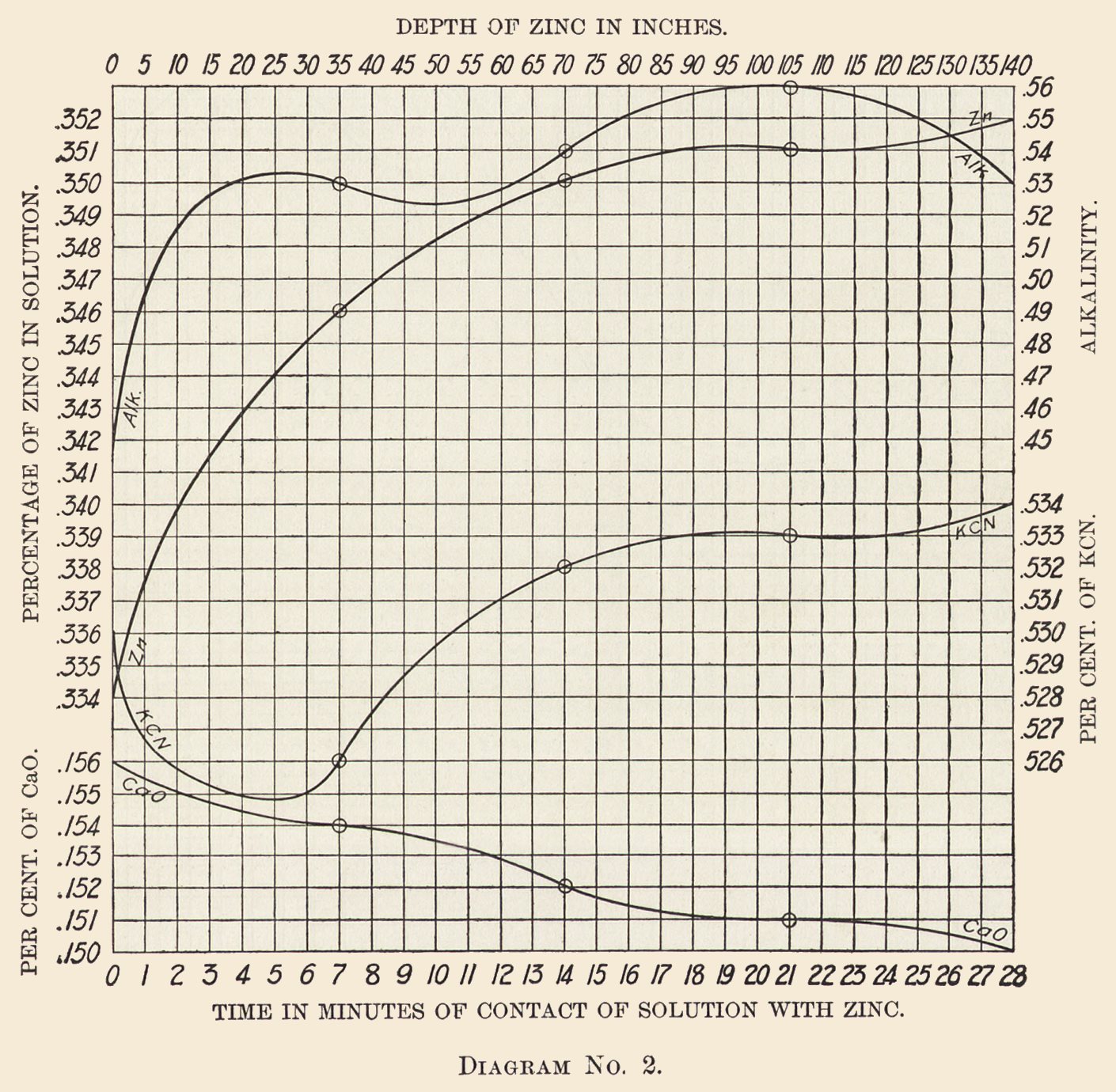
Diagram No. 2 shows the average zinc, cyanide, lime, and alkalinity curves for the same solution and during the same period of time.
It will be noticed from Diagram No. 2 that the lime is partially precipitated or filtered out in passing through the boxes. In this case it enters the box at 0.156% and leaves it at 0.15%, the precipitation being quite regular, in fact almost a straight line on the diagram.
We use lime as a neutralizes which to a slight extent passes into solution. The highest I have found it is 0.19%, while the average percentage held in solution is but 0.12%.
Cyanide Curve: The cyanide enters at 0.53% and immediately falls to 0.526%, then rises steadily to 0.534%. It is not at all unusual to find the free cyanide higher in the solution leaving the boxes than on entering; still it is not the rule.
Our experience with solutions of this strength is that there is no appreciable loss of cyanide in passing through the boxes. Sometimes the issuing solutions are higher, sometimes lower, while the average shows about the same strength of cyanide in the solutions issuing from the boxes as contained in the solutions entering them.
Zinc Curve: The zinc carried in the solution entering the boxes is 0.334%. The curve rises rapidly until 97% of the gold is precipitated and then flattens. Tabulating the results we have the following:
After the solution has filtered through 35 in. of zinc, 0.012% Zn is dissolved and 92.4% of the gold is precipitated
after 70 in. of zinc, 0.004% Zn is dissolved and 4.7% of the gold is precipitated
after 105 in. of zinc, 0.001% Zn is dissolved and 1.83% of the gold is precipitated
after 125 in. of zinc, 0.001% Zn is dissolved and 0.17% of the gold is precipitated.
The total, 0.018% of Zn, equals 0.30 lb. of zinc, while 99.10% of the gold is precipitated, or 0.364 lb. adv. of zinc per ounce of gold precipitated, or 5.31 troy oz. of zinc used to precipitate 1 oz. of gold and 0.1 oz. of silver.
Alkalinity Curve: We invariably find an increase of alkalinity in cyanide solutions while passing through the boxes. (The alkalinity is reported in terms of sodium hydrate, using phenolpthalein as indicator.) The alkalinity rises rapidly with the precipitation of the gold and falls again toward the end of the box, usually leaving it at about the strength it entered, or very little in excess.
The following partial analyses of these solutions were made at the end of the experiments, excepting KCN, which was determined separately each day:
| No. 1. | No. 2. | No. 3. | No. 4. | No. 5. | |
|---|---|---|---|---|---|
| Per Cent. | Per Cent. | Per Cent. | Per Cent. | Per Cent. | |
| KCN | 0.5300 | 0.5260 | 0.5320 | 0.5330 | 0.5340 |
| HCN | 0.0269 | 0.0269 | 0.0290 | 0.0332 | 0.0373 |
| KCNS | 0.0388 | 0.0389 | 0.0389 | 0.0388 | 0.0386 |
| K4Fe(CN)6 | 0.0580 | 0.0690 | 0.0810 | 0.0840 | 0.0880 |
| CaO | 0.1560 | 0.1540 | 0.1520 | 0.1510 | 0.1500 |
| Zn | 0.3340 | 0.3460 | 0.3500 | 0.3510 | 0.3520 |
No ferricyanide or alkaline sulphides were present.
No. 1 is the average solution entering.
No. 2 the solution after filtering through 35 in. of zinc.
No. 3 the solution after filtering through 70 in. of zinc.
No. 4 the solution after filtering through 105 in. of zinc.
No. 5 the solution after filtering through 140 in. of zinc.
This particular solution had been in use on telluride ores for two and one-half years. It was of course kept up to the proper volume and strength, but no solution had been wasted from the mill. During this period 127,327 oz. of fine bullion had been dissolved by the solution and precipitated from it by means of zinc.
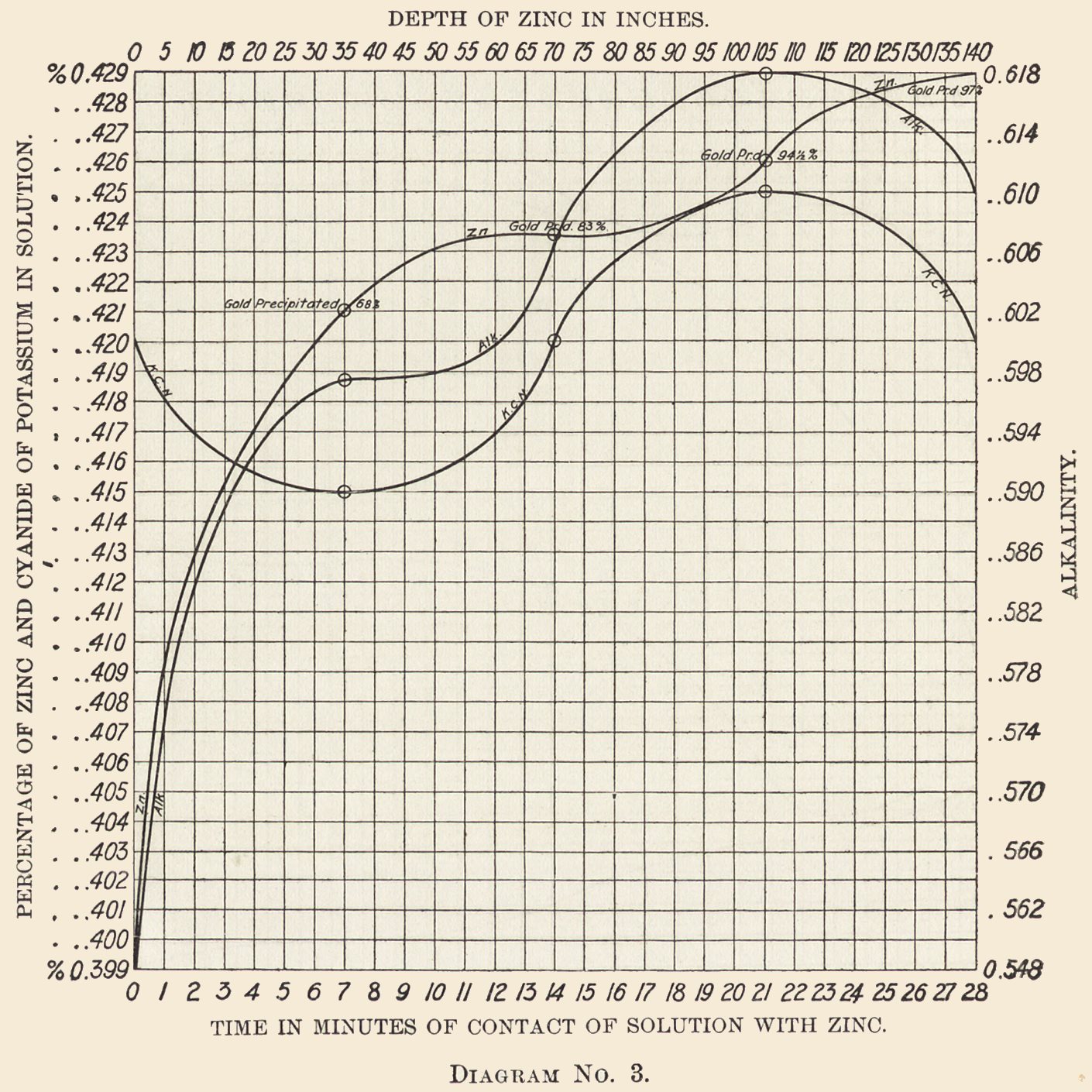
Diagram No. 3 shows that a strongly alkaline liquor, while causing a rapid solution of the zinc, does not precipitate the gold in proportion. This diagram is from 24 hours' run of a strongly alkaline solution. The zinc in solution entering the box was 0.399%.
After the solution had passed through 35 in. of zinc, 0.022% of Zn is dissolved and 68% of the gold is precipitated.
After 70 in. of zinc, 0.003% of Zn is dissolved and 15% of the gold is precipitated.
After 105 in. of zinc, 0.002% of Zn is dissolved and 11.5% of the gold is precipitated.
After 140 in. of zinc, 0.003% of Zn is dissolved and 2.5% of the gold is precipitated, making a total of 0.030%, or 0.60 lb. of the zinc dissolved, and 97% of the gold precipitated.
Cyanide Curve: The entering solution carries 0.42% KCN, falls in seven minutes to 0.415%, rises in the next seven minutes to the entering strength 0.42%, continues rising to 0.425%, and falls in the last seven minutes to 0.42%, the strength at which it entered.
Alkalinity: The solution enters at 0.548%, immediately rises to 0.597%, continues rising until it reaches 0.018%, and then falls to 0.61%.
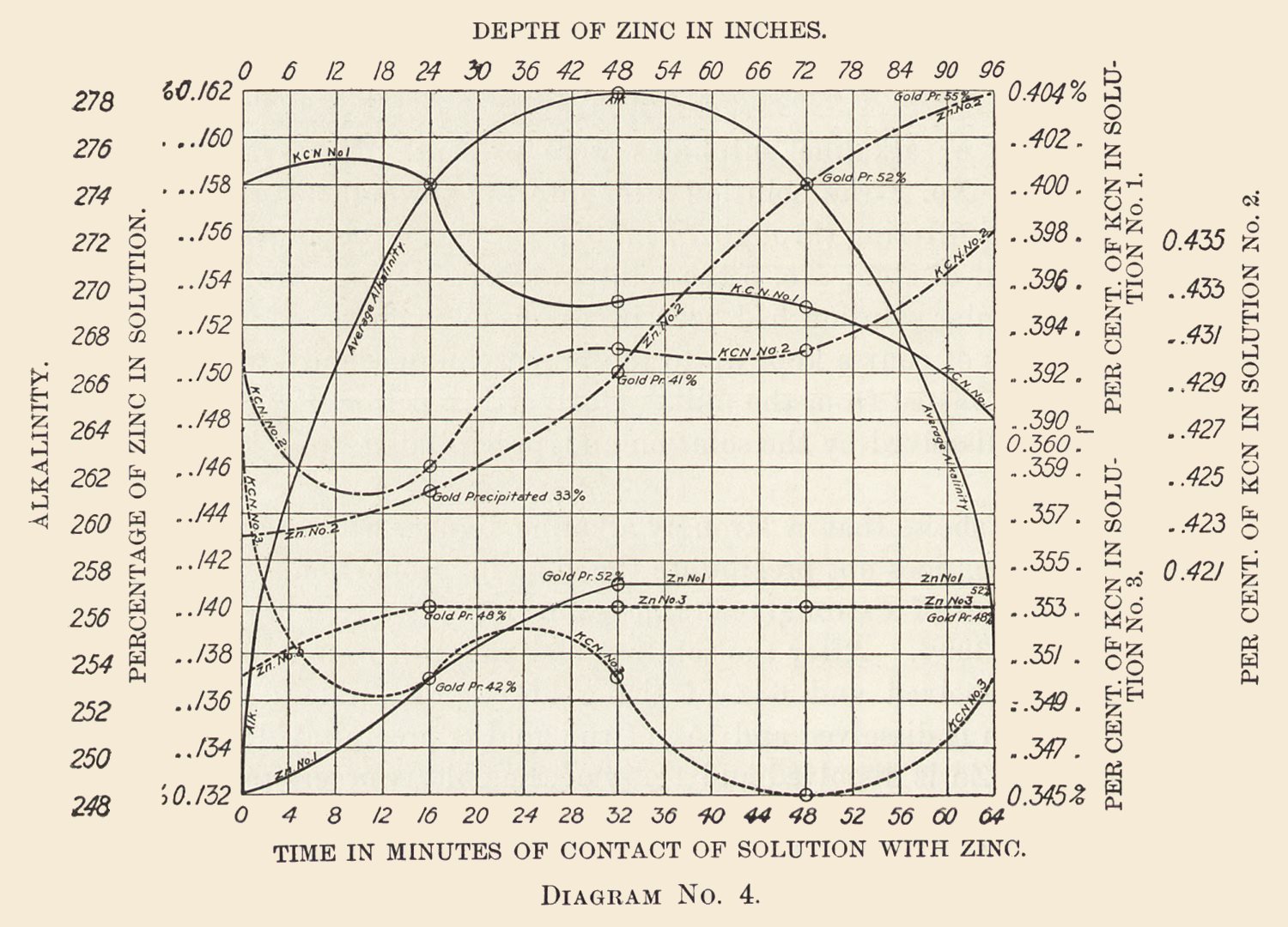
Diagram No. 4 gives some horrible examples of precipitation, or rather lack of it, but nevertheless it is instructive. These solutions had percolated through acid ores and were too low in alkalinity.
The addition of caustic soda to the solution immediately set matters right. Cyanide of potassium would have accomplished the same result, but in these solutions caustic soda was preferable.
The solution No. 1 contained 0.132% zinc on entering.
After passing through 24 in. of zinc, 0.005% of Zn was dissolved and 42% of gold was precipitated.
After 48 in. of zinc, 0.004% of Zn was dissolved and 10% of the gold was precipitated.
After 72 in. of zinc, no Zn was dissolved and no gold was precipitated.
After 96 in. of zinc, no Zn was dissolved and no gold was precipitated.
A total of 0.009%, or 0.18 lb. of zinc dissolved and 52% of gold precipitated.
In No. 2 the entering solution contained 0.143% Zn.
After passing through 24 in. of zinc, 0.002% of Zn was dissolved and 33% of the gold was precipitated.
After 48 in. of zinc, 0.005% of Zn was dissolved and 8% of the gold was precipitated.
After 72 in. of zinc, 0.008% of Zn was dissolved and 11% of the gold was precipitated.
After 90 in. of zinc, 0.004% of Zn was dissolved and 3% of the gold was precipitated.
A total of 0.019%, or 0.38 lb. zinc dissolved and 55% of gold precipitated.
Here the volume of solution passing was too great or the precipitation box too short, which amounts to the same thing.
Anyhow the precipitation continues fairly regular, and given longer contact with the zinc would probably have been satisfactory. These particular solutions averaged about 0.25 oz. of gold per ton as they entered the boxes.
In No. 3 the entering solution contained 0.137% Zn.
Which after passing through 24 in. zinc showed 0.003% of zinc dissolved and 48% of the gold precipitated.
But after passing through 48, 72, and 96 in. of zinc no zinc was dissolved nor gold precipitated, the total of the experiment remaining 0.003% or 0.06 lb. zinc dissolved and 48% of the gold precipitated.
The gold on entering was 0.23 oz.; on leaving boxes, 0.12; alkalinity on entering 254, middle 294, end 274.
Our general practice shows a consumption of 0.92 lb. of zinc for each ounce of fine bullion produced. Of this amount not more than 40% is dissolved, the remaining 60% being removed from the boxes with the precipitate at each weekly clean up, and owing to its fine state of division and richness in gold we find it preferable to destroy the zinc and recover the gold.
With normal solutions carrying about 1 oz. of gold per ton in a 0.5% cyanide solution, not more than 0.33 lb. of zinc is dissolved for each ounce precipitated. This distinction between the zinc dissolved and the total zinc used in precipitation should be carefully noted; all the figures of critics that I have seen are apparently based on all the zinc being dissolved.
Solutions rich in gold precipitate far more readily than weaker ones, other things being equal. When the solutions pass 1.5 oz. per ton the gold on the shavings assumes a yellow color, and passing 2 oz. and upward is usually golden yellow in the first compartment, shading off in the following compartments to the usual black-colored deposit that collects on the zinc in ordinary precipitation from poor solutions.
The precipitation of the precious metals by means of zinc is very often supposed to be a weak point in cyaniding, many failures of the process being attributed solely to this cause. I would not call it a weak point, but on the contrary a very strong one, requiring, it is true, careful attention, proper manipulation, and solutions in good working condition; given these, it is the cheapest and best method of gold precipitation known.
The essential conditions for good precipitation are:
First, clear solutions,
Second, moderately alkaline solutions containing free cyanide of potassium,
Third, boxes properly prepared with the zinc.
As regards the first requirement, dirty or slimy solutions coat the zinc and impair precipitation. This sometimes happens with solutions apparently clear, but which on careful examination are found to contain matter in suspension; it is therefore good practice to have large settling tanks to receive the solutions draining off from the leaching tanks, these settlers answering two purposes, namely, to settle out suspended matter and to equalize the flow through the precipitation boxes, which latter is also an important feature.
As a second condition precedent to good precipitation, we must have a moderately alkaline solution, otherwise the zinc is but little acted on and soon becomes passive, while on the other hand a strongly alkaline solution is, as we have already seen, detrimental in other ways; again, free cyanide is an essential condition for good precipitation, though in moderately alkaline solution, fairly free from metallic salts, a very small amount of cyanide will suffice, provided long contact is given between the solution and the zinc.
It goes without saying that the solutions must be kept free from the accumulation of metallic salts, a matter often attained by the judicious use of lime.
Third, when the zinc shavings are used they should be cut in as fine threads or filaments as possible, having, however, sufficient substance to hold together and not break in handling. These shavings should be pressed down evenly in the boxes and with some little force. When properly packed the shavings will occupy but 3% of the space, leaving 97% interstitial space for passage of solution.
The boxes are preferably prepared every 12 hours, all the zinc in the first three compartments being stirred up to detach adhering precipitate and foreign matter and then filled with zinc pressed down as above described; finally, the zinc-boxes should be completely cleaned up every week to keep them at their best, for it must be remembered these boxes are huge filters, each one in the Metallic Extraction Works passing 1,000 tons of solution per week, and if this solution deposits but 2 oz. per ton (almost an infinitesimal amount) we have about 2 oz. foreign matter to each ounce of gold precipitated; very often there is fully that amount of lime extracted from the solutions in passing through the boxes, while manganese and various other substances are also deposited; hence the importance of freeing the zinc from such deposits, as pointed out above.
Continuous samples of the solutions entering and leaving the boxes should be taken and assayed every 12 hours, though to the experienced eye the evolution of hydrogen from the solution passing through the boxes clearly indicates the condition of the precipitation; the assays, however, give the accurate check on the work.
Perhaps in no part of the process is there wider differences in practice than there is in refining the precipitates. The method we have worked up, and have had in use for about two years, is as follows:
The precipitate is placed in cast-iron pans, which are subjected to a dull red heat in a muffle furnace.
When this roast is complete the precipitate is treated with hydrochloric acid in pans having mechanical agitators.
The residue is then washed, air-dried on the filters, fluxed, and replaced in the roasting pans for thorough drying, and is afterward smelted.
The bullion runs 950 to 980 fine, and is quite homogeneous and is easily sampled; in fact, the mint returns check very closely our sampling and assaying.
The cyanide process when first introduced in Colorado was advertised as simplicity itself. I quote from an advertisement:
"The process consists of pulverizing the ores to a fineness of about 50-mesh and then subjecting the same to a very dilute solution of cyanide. A few tubs, especially constructed according to our diagrams for triturating, settling, and filtering, is all that is required extra in connection with any kind of pulverizing stamps or rolls."
The process is even now usually looked upon as a cheap and crude method of gold extraction, particularly applicable to tailings and low grade-ores. Some stock writers even go so far as to tell one in advance the extraction that can be made by cyanide (which is never above 80%, if my memory serves me), and this quite irrespective of the value of the ore, or conditions in which the gold exists; hence they argue in a circle and show that low grade ore can only be worked at a profit.
In the works of the Metallic Extraction Co. I have applied cyanide to the treatment of fairly rich ores, and more particularly to roasted ores on an extremely large scale, and in the latter at least I believe we have led the way and established the process as second to no other wet method of gold extraction.
Engineering Magazine, September 1894, page 814.